
Fuel, Lubricant and Coolant Data
Notes on Cleaning Up Fuel System Deposits
Brake Fluid Performance and Comparisons
Lubricants:
Oil Additive Information Links
Fuel:
Gasoline Recommendations for Turbo Engines
Reformulated (Oxygenated) Gasoline Areas
Car Storage, Fuel Degradation and Engine Deposits
Notes on Cleaning Up Fuel System Deposits
Injector Deposit Problems. [Import Car Magazine, Mar '03 by Larry Carley]
To save a few pennies per gallon and to increase the competitive and/or profit margin of gasoline, some suppliers have cut back on the amount of detergent they add to their fuel or have switched to cheaper and less effective additives. Commonly used deposit-control additives include polysibutylamine, polyisbutylene succinimide and polyisobutylene phenylamine. But these same additives also can build up on intake valve stems causing them to stick. To prevent this from happening, additional additives called fluidizers must also be added to the fuel. But, over time, these can contribute to the formation of combustion chamber deposits that raise compression and the engine's octane requirements. Dirty injectors lean out the fuel mixture and contribute to lean misfire, hesitation and even detonation.
Cleaning should restore like-new performance. One of the best additives is polyetheramine. It keeps injectors, valves and combustion chambers clean without the help of any additional fluidizers - but it costs more than twice as much as the other commonly used additives. How much additive does it take to provide an adequate level of protection? Industry sources say the recommended level is about 1,000 parts per million (ppm) of dispersant-detergent in the fuel - which costs the gasoline supplier less than a penny a gallon. Even so, as much as 85% of the gasoline that's being sold contains only one-tenth of the recommended dosage, or only 100 ppm of additive. Consequently, using cheap gas contributes to the formation of injector deposits. ..The classic symptoms of dirty injectors include lean misfire, rough idle, hesitation and stumbling on light acceleration, a loss of power, and higher hydrocarbon (HC) and carbon monoxide (CO) emissions.
Gasolines with Detergents. In the United States, the new Top Tier gasolines are certified to have adequate detergent levels to remove intake system deposits as determined by the Top Tier group of GM, BMW, Honda, and Toyota. Since the minimum additive performance standards were first established by EPA in 1995, most gasoline marketers have actually reduced the concentration level of detergent additive in their gasoline by up to 50%.As a result, the ability of a vehicle to maintain stringent Tier 2 emission standards has been hampered, leading to engine deposits which can have a big impact on in-use emissions and driver satisfaction.Top Tier fuels are recognized by the vehicle manufacturers as having the most effective additives and in the highest concentrations. Gasoline retailers must meet the high Top Tier standards with all their grades of gasoline (not just premium) to be designated a Top Tier supplier. In addition, all the gasoline outlets carrying the brand of approved gasoline also must meet the same standards. Gasoline retailers who are currently on the Top Tier list include Chevron, Chevron-Canada (B.C. only), Texaco (Chevron supplied only), Conoco, Entec Stations, Kwik Trip/Kwik Star, MFA Oil Company, Phillips, QuikTrip, Shell, The Somerset Refinery and 76. See their website at http://www.toptiergas.com/
Fuel Additives
[Mark Burns] Some of the high end aftermarket fuel additive packages are very effective at removing deposits in the fuel injectors, intake ports, intake valves, combustion chambers, and exhaust valves. One of the best products available is the STP Complete Fuel System Cleaner. It can out perform Techron and other Techron-based products like Slick 50 and Gumout Regane with respect to intake valve and combustion chamber clean up. These aftermarket packages can remove any and all deposits in the system if used at sufficiently high or repetitive dosages although there is some risk of crankcase lube contamination. All deposits in the system are carbonaceous. The structure of the deposits in the injectors is different from the structure of the deposits on the valves is different from the structure of the deposits on the combustion chamber, but all are carbon based. The chemistry that is able to remove each type of deposit depends on the temperature profile that each area sees and the thermal stability of the detergent in the additive package. Since October 1, 1993, all gasoline marketers are required by the federal government, according to the Clean Air Act Amendments, to deliver a minimum level of deposit fighting additives in their fuel. Up to that time, deposit control additives had been used to differentiate branded gasoline. The major gasoline marketers usually added at least a competent package to all of their grades. The lesser known brands rarely added any additives at all. About thirty percent of the gasoline sold were not additized. The minimum level of deposit control performance that all gasoline must meet are two standard deposit control tests-the ASTM D 5500-97 BMW intake-valve deposit test and the ASTM D 5598-95 Chrysler 2.2-liter port-fuel-injector test using a test fuel that encompasses the sixty-fifth percentile of U.S. fuel severity parameters. This federally mandated requirement for gasoline has established a lowest common denominator for deposit control performance.
Gasoline additive suppliers have found ways to minimize additive dosage and beat these two tests. The result has been an overall reduction in the deposit control performance of U.S. gasoline. While a fuel marketer may have data demonstrating that their specific additive package once passed the BMW intake valve test and the Chrysler port fuel injection test, there are no guarantees that the gasoline they are marketing will provide adequate performance in any consumer's engine. There are many commercial gasoline, available in the market today, including some major national brands, that, when tested in fleet test vehicles representing various drivetrain configurations, have developed more than 1000 milligrams on the intake valves. This is ten times the maximum amount of deposit allowed for passing the BMW intake valve test. There is even some evidence that the very low levels of deposit control additives being used by some gasoline marketers actually make the base gasoline create greater levels of intake valve deposits in the average engine than the unadditized gasoline would. It could be suggested that more consumers on the road today could benefit from the occasional use of a high quality aftermarket additive package than ever before. In some gasolines on the market, use of a high quality gas treatment package would certainly be advised if a driver wants a greater degree of certainty that their engine will be kept clean.
[Paragraph from Dave Stevens:] The prevailing wisdom seems to be that BG44K is an excellent, but very strong cleaner, really only appropriate for use on badly carboned/neglected engines, especially considering its cost. I mean, you don't need oven cleaner to do the dishes. You can try getting it through a friendly shop or auto supplier or you can try getting it from one of the auto e-tailers. Other top end/injector cleaners you may want to try are AMSOIL PI (through distributors like our Paul S), Chevron Techron (concentrate) and Redline SI. That's more or less the order of preference I've seen. Some of the house branded injector cleaners (like GM and Mazda) are reportedly as strong as BG44K. There are many other injector cleaners out there, some may be okay, especially for routine use. Just be sure to avoid the cheaper, older solvent based cleaners that might attack the fuel system and injector seals. Periodic use of additives (like every year or so) should be the most that's necessary if you use quality gas, your engine is properly tuned and you do a reasonable amount of highway driving. If you buy cheaper gas with minimal additives or lower octane than the engine requires or if you do strictly stop and go driving, then more frequent use (like every few months) may be required.
Other Approaches to Deposit Control
There are other approaches to the fuel system cleaning besides adding aftermarket fuel additives. One is to use water as described in the FAQ link, one is the fuel tank/vacuum induction fuel system cleaning approach that many quick oil change places use and one is the high tech, expensive (some of these machines cost $4000), and complicated machine approach. Water is a great deposit remover. It is just like steam cleaning the combustion chamber.
Unfortunately, the heavy components of fuel and fuel additives are liquid during the combustion process and don't get completely burned. About 25% of the active fuel additive components (the oligimeric detergent and fluidizer components) end up in the crankcase. These components may or may not be compatible with the oil. As you can imagine, water does not burn. It may leave through the exhaust valve as steam or it may end up in the crankcase. Do you want 25% of the total amount of water used in the cleaning process to end up in the crankcase? You can change the oil right away or you can run the engine long enough and at a sufficient speed to distill off the water. After any of the serious fuel system cleaning, the oil should be changed anyway. You can draw your own conclusions about the effects of water in the crankcase and the prospects of getting all of it out, but you can clean up the engine just as effectively without the use of an oil insoluble actor. There are some systems out there that use water in the fuel system cleaning. I think these systems usually employ some kind of very expensive machine. I don't think they clean more effectively than the fuel tank/vacuum induction fuel system cleaner. Cars are sensitive to deposits, but not that sensitive.
The fuel tank/vacuum induction fuel system cleaner cleans injectors, intake valves and the combustion chambers through the action of the bottle of additive poured into the fuel tank. The vacuum inducted intake system cleaner is added through a vacuum line behind the throttle plate. The purpose of the intake system cleaning is to remove deposits left by the PCV and the EGR as well as aiding in cleaning up the intake valves, ports, and combustion chambers. One brand that is very effective and provides a high quality product is C.A.T. Products makers of Run Rite.
The tool used to induct the intake system cleaner into the vacuum line is usually a metal bottle with a tube in it that connects to a hose with a fitting on one end to connect to a vacuum line close to the throttle plate on the vacuum side. There will be no problem as long as the engine is running, it will suck in the cleaner. If the engine stops but the fluid keeps flowing, you can hydrolock the engine and damage valves, rods, pistons and gaskets. These tools often have a valve in line and a clear portion in the hose after the valve to adjust the feed and monitor the flow so that it is a steady drip. The fluid usually used in the bottle is an air intake/throttle plate cleaner package. Only the additized fuel in the tank goes through the injectors. Based on what I have seen, this should work as well as any injector cleaning scheme on the market. Fuel injectors deposits are not as much of a problem now as they were a few years ago. New injectors are more resistant to deposits and most gasolines, as poor as they are at controlling most deposits today, still can keep injectors (and carburetors) clean. STP Fuel System Cleaner works very, very well. Two bottles should have them spotless and will clean the valves, ports, and the combustion chambers. The fuel tank/vacuum induction fuel system cleaning makes an immediate difference in the way the car runs. It must have something to do with the EGR and PVC deposits. You can also try to replace the PCV. The beauty of the vacuum induction fuel system cleaner approach is that it doesn't require a degree in mechanical engineering and a master mechanic certification to operate: pour a bottle in the tank then find a vacuum hose and suck a bottle of the intake system cleaner into the intake. I don't think the systems that utilize the expensive machines actually clean the fuel system any more thoroughly.
The problem with the machine systems hooked up to the fuel rails is that they can not clean the parts of the system that the fuel does not get to. Cleaning through port fuel injectors can clean the injectors, intake ports, intake valves, and combustion chambers. Cleaning through the vacuum line cleans the entire intake manifold, intake ports, intake valves and combustion chambers while the fuel additive added to the tank cleans the injectors, intake ports, intake valves, and combustion chambers, albeit at a slower rate as the fuel in the tank is burned over about 350 miles. I think the fuel tank/vacuum induction fuel system cleaner approach may, in fact, provide a more thorough cleaning. I personally do not believe that the expensive and complicated machine/high pressure systems have any advantage over the simple approach that we are using. They do, however, have a major drawback in that there are more things to go wrong. The technician has to disconnect the fuel pump and connect to the fuel rail. There is big potential for disaster with this approach if the technician is not highly trained. It is pretty easy to pull off a vacuum line and suck in the cleaning solvent. If the vacuum line is not reconnected properly, the car will not run, but it is easy to diagnose and fix. It is also unlikely to burn the car up if the technician doesn't do something right.
The walnut shell blasting can be done without removing the head. It is a fairly difficult operation and requires the right equipment. You also have to make sure you remove all of the residual walnut shell. All in all, the aftermarket fuel additive packages or the fuel tank/vacuum induction fuel system cleaning are probably the least intrusive approaches.
Current Coolant Chart. [Editor] See the coolant chemistry chart from Old World Industries
Here are some links to informative sites about coolant and antifreeze topics:
http://www.vanagon.com/info/articles/coolants.html
Notes from the Jaguar Lovers Club by Jim Crider. Here's a response from someone who designs vehicle cooling systems for a living ...... (that would be me):
Strictly looking at the heat transfer coefficient, straight water is the way to go. HOWEVER... straight water has its problems, notably a lack of certain additives that prevent cavitation of the water pump at high speeds; corrosion of the various metal bits present in all engine cooling systems; surfactants to lower the surface tension of the coolant (allowing it to wet the surfaces of the coolant passages better); anti-foaming agents to keep the surfactants from making big bubbles; and freeze point and boiling points that are closer together than a mix of coolant and water.. All these are present in antifreeze/coolant. The surfactants and anti-foaming agents are present in Redline Water Wetter. Water Wetter has limited to no benefit in a system using a commercial coolant -- it's simply adding more of something already present in sufficient quantity.
There are two types of base coolant stock available right now: Ethylene glycol (EG) and propylene glycol (PG). Currently, no engine manufacturers selling product in the US recommend PG (sold by Arco as Sierra brand coolant), most caution against it (check your owner's guide). PG has a higher boiling point than EG (straight), but has a lower heat-transfer coefficient. EG coolants also come in several flavors, depending on the additive package (more below). BTW, PG isn't truly non-toxic. It's LESS toxic than EG, but PG coolant contains various and sundry additives that aren't really good for you. Basically, the less-toxic claim only applies if you pour the stuff straight out of the bottle and onto the ground. Don't bother with it. And treat *any* used coolant as low-level hazmat. Small amounts can be disposed of in sanitary sewer systems, but you're better off making nice with the operator of the neighborhood quickie lube place, who will be able to take it off your hands and get it into the recycling stream, sometimes for a nominal fee.
The green-dyed EG conventional coolant we all know and love has an additive package based around a silicate (and sometimes also phosphate) based anti-corrosion additive. It's well-established and does a good job. It can go 5 years/50K miles without worry.
A few years ago, someone thought a long-life coolant (original plan: life of vehicle) would be a Good Thing. This lead to Organic Acid Technology coolant (OAT), which is marketed as DexCool by GM and has been factory-fill in their products (except C4 Corvette -- not sure about C5 Corvette) since 1995. It's the orange or orangy-red stuff. Someone along the line decided the word acid was a Bad Thing to try to sell, so OAT was recursively changed to Organic Additive Technology. It can go 5 years/100K or 150K miles -- provided it's not mixed with other coolant. OAT has less cavitation resistance than silicate-based coolant, and can attack certain sealing materials, so it's not a good idea to convert a green-coolant car over to OAT unless the manufacturer says it's okay. OAT also has a tendency to stain translucent plastics in things like overflow bottles and pressurized de-gas bottles with a funky brown crud. Oh, and OAT from onemanufacturer isn't necessarily compatible with OAT from a different manufacturer. Texaco is GM's OEM supplier and is licensed to use GM's DexCool trademark on their aftermarket packaging. I'm not aware of any other company being licensed to do so.
Many European automakers use a hybrid of OAT -- HOAT (Hybrid Organic Additive Technology -- clever, huh?), which is the OAT package with a small amount of silicates added to increase the cavitation resistance and make it less aggressive against those seals and gaskets. This is often pale yellow in color. DaimlerChrysler is using it in several car lines now, too, notably the LH sedans and the new minivans and PT Cruiser. This stuff seems to offer pretty much the best of both worlds -- it's not quite as long-lived as straight OAT, but it is much better behaved in operation than OAT, much like conventional coolant. It is now sold by Valvoline under the Zerex G-05 label and by Texaco as Havoline Custom-Made.
Note that these three different additive packages are not really cross-compatible. No, they won't eat the insides of your radiator if you mix a little of one in with another in a pinch, but you'll be better to get the system flushed out and a fresh mix of 50/50 whatever your car needs put back into it. In my own cars, I run a 50/50 EG/W coolant mix. I happen to own cars that take conventional coolant, but if I owned a car that came with OAT or HOAT from the factory, I'd likely stay with it. The anti-corrosion additives, in particular, leave residues on the walls of the various coolant passages (that's how they work -- the residues coat the base metal and prevent corrosion), and it's tricky to convert an engine that's been run with one style of package to use another package and get the full benefit. Switching from conventional to OAT, for instance, requires a mild acid flush of the cooling system after removal of the conventional coolant and before pouring in the OAT if the long-life corrosion benefits of the OAT coolant is to be realized. Just pouring the OAT in after draining the conventional won't gain the full measure of added coolant life the OAT marketers (notably Texaco) like to use as selling points. [Note from Texaco regarding rumored incompatibilities:] We have seen the statement many times that On '93 and older GM models, use of this antifreeze is discouraged because its chemical ingredients can interact with the copper-soldered joints inside the radiator. It was even in Motor Trend recently, which has spread the rumors further. The statement is not true. Havoline Extended Life Antifreeze DEX-COOL can be used in any car including 93 and earlier model GM cars without any problems.
It is true that some older GM cars used a high lead solder in copper brass radiators where their newer cars are all aluminum. However, Havoline Extended Life Antifreeze DEX-COOL protects the high lead solder very well, there is no detrimental interaction with the solder or radiator, and there is no need to be concerned. Indeed, although GM decided to be conservative in not recommending DEX-COOL for all older cars, Texaco has recommended Havoline DEX-COOL for ALL cars, and stands behind the product in ALL cars.
[Stephen Goldberger] It is claimed by the manufacturer to leave a thinner inhibitor layer on the metal, resulting in improved heat transfer, and it is claimed to be less abrasive to the water pump seals. The inhibitor in DexCool is a non-silicate formulation, more along the lines of the European sebacic acid practice, but it is not the same. The 100,000 mile or 5-year recommended change interval is only true for vehicles which had DexCool as original fill; otherwise, it is recommended that the original factory change interval be adhered to.
[Notes from Underhood Service Magazine, Oct 2005] There are essentially three basic types of coolants:
Traditional North American green antifreeze, the original universal formula that everybody used until the introduction of extended-life coolants 10 years ago. The fast-acting silicate and phosphate corrosion inhibitors provide quick protection for bare iron and aluminum surfaces, and have a proven track-record of providing trouble-free service in virtually any vehicle application (domestic, Asian or European). But the short-lived nature of the corrosion inhibitors means this type of coolant should be changed every two to three years or 30,000 miles (though some products now claim a service interval of up to 50,000 miles with improved chemistry).
OAT-based extended-life coolants. OAT stands for Organic Acid Technology, and includes such ingredients as sebacate, 2-ethylhexanoic acid (2-EHA) and other organic acids, but no silicates or phosphates (except in the case of Toyota's pink extended-life coolant, which adds a dose of phosphate to its extended-life OAT-based antifreeze). OAT-based coolants are usually (but not always) dyed a different color to distinguish them from regular North American green antifreeze. GM's OAT-based Dex-Cool is orange. Volkswagen/Audi uses a similar product that is dyed pink. But Honda has an extended-life OAT coolant that is dyed dark green and does not contain 2-EHA. The corrosion inhibitors in OAT coolants are slower acting but much longer-lived than those in traditional North American green coolants. Consequently, OAT coolants typically have a recommended service life of five years or 150,000 miles. OAT corrosion inhibitors provide excellent long-term protection for aluminum and cast iron, but may not be the best choice for older cooling systems that have copper/brass radiators and heater cores. It depends on the formula. One ACDelco spokesman said they do not recommend Dex-Cool for older vehicles with all-iron engines and copper/brass radiators.
Hybrid OAT coolants, also known as G-05. This formulation also uses organic acids, but not 2-EHA (different organic acids are used). Hybrid OAT coolants add some silicate to provide quick-acting protection for aluminum surfaces. Silicate also helps repair surface erosion caused by cavitation in the water pump. Hybrid OAT coolants are currently used by many European vehicle manufacturers as well as Ford and Chrysler.
[Notes from Motor Magazine, Aug 2004]
The DexCool designation means the coolant passes General Motors performance testing. Although DexCool is not a specific formula, all three brands that have the label (Texaco Havoline, Prestone Extended Life and Zerex Extended Life) are somewhat similar. In particular, they're OAT coolants, but the similarities go beyond that basic description. All DexCool-approved coolants to date use two organic acid rust/corrosion inhibitors, one called sebacate, the other called 2-EHA (which stands for 2-ethylhexanoic acid). These organic acids are very stable and last a long time, although they take thousands of miles to become fully effective in protecting coolant passages. GM recommends a DexCool change every five years or 150,000 miles, whichever comes first. Because most people drive 15,000 to 20,000 miles a year, that translates to a five-year replacement interval. As noted, the thousands of miles required to protect metal is an important trade-off for that longer life. Although like conventional coolants, OATs also contain other inhibitors, for targeted protection. The inhibitor 2-EHA works well in hard water and is more effective than sebacate at lower pH levels (when the coolant moves from the alkaline end toward the acid side), particularly for cast iron. Well, GM has a number of cast-iron engines. When there's a low coolant level in the coolant passages, the exposed cast iron rusts. Apparently, that rust is washed away later by flowing coolant, and is deposited in the heat exchangers. It eventually produces the rust powder problems that have been so widely observed... The inhibitor 2-EHA poses another issue: It's a plasticizer (softens plastic), so it has been blamed for coolant passage gasket leakage. Softening (and the resulting distortion) was reported by Ford, which encountered gasket leakage problems when it tested a DexCool-type formula on its V8 engines. Ford also saw similar issues with other gasket materials... Ford and Chrysler Group use G-05, a low-silicate, no-phosphate formula long specified by Mercedes, even for its passenger car diesels.
What is G-05? It's called a HOAT (for hybrid organic acid technology) that today serves for extended intervals, typically 5 years/100,000 miles. Like conventional Euro coolants, it's a low-silicate, no-phosphate formula designed to pass European hard water tests. The reference to OAT in HOAT is for an organic acid inhibitor called benzoate, which actually has been used for many years in almost every American, Japanese and European conventional coolant except what we now call OAT. Honda and Toyota use a new extended-life OAT coolant-made with sebacate as the only organic acid and no 2-EHA. Sebacate isn't quite as effective in combating corrosion at lower pH levels, but because that's more of a cast-iron issue, it apparently didn't concern the Japanese. Both Honda and Toyota do continue to avoid silicates, but add a dose of phosphates to provide fast-acting aluminum protection, particularly to recoat the water pump after cavitation erosion/corrosion....One of the issues that may arise is the use of an aftermarket replacement radiator or heater core made of copper-brass with lead solder. We have in previous articles pointed out that today's coolant inhibitor packages contain a small amount of copper-brass protection, but may provide little protection if a radiator is made with high-lead solder. Results of industry standard tests of the new Toyota extended-life coolant now show a substantial weight loss (corrosion), both in a 50-50 mix and in a 33% coolant mixture (solder corrosion is much greater in this more diluted solution). If you have to change a radiator or heater core, use aluminum. Or, if it's an older car and the owner wants the lowest-cost radiator, you might procure a soldered-together copper-brass unit. Conventional American coolant should provide better protection against solder corrosion, which can result in radiator tube restrictions and leaks. But no coolant provides perfect protection.
Makeup Water. [Tip from Prestone and consistent with other manufacturers' recommendations] We would consider the order of preference for water to be as follows:
- 1st choice: Type IV water- Both demineralized & deionized.
- Next choice: Distilled water
- Next choice: Bottled water (like the type at a grocery store)
- Last choice: Tap water
Note that the "deionized" water available at the supermarket may be merely softened with sodium replacing some of the other ions. Distilled is a better choice. Note as well that a prevalent urban legend cautions against "distilled water as too aggressive"; in a coolant mixture this assertion is ridiculous.
Lubricants:
Oil Information Links. Check out these great links to find out all/more than you ever wanted to know about oil:
Two excellent book references are:
- Synthetic Lubricants and High-Perfomance Functional Fluids, 2nd Edition, Leslie R. Rudnick and Ronald L. Shubkin, editors, 1999, published by Marcel Dekker, Inc. New York
- Automobile Engine Lubrication, Alphonse Schilling, 1972, Scientific Publications Ltd., Broseley, Shropshire, England translated from French.
Synthetic Oil Notes. [Tips from Geoff Williams, edited] I have spent the last 6 months learning about which oils will work best in my new VW TDI engine. In that process I have come across a wealth of information. I'll state the following hopefully in a straight forward manner (BTW my wife is now the primary driver of our '86 240 wagon).
1. Oil Groupings. American Petroleum Institute (API) has defined five groups (I through V) of base lubricating oils. The system established three groups (I-III) of paraffinic base oils based on levels of saturates, sulfur, and viscosity index (VI), as well as PAO-based oils (IV) and ester, PAG and other oils (V). Recent developments include the informal (non-API) addition of "+" subcategories and a new Group VI:
- Group I. Mineral oils refined using the historical techniques (i.e., solvent extraction of aromatics, solvent dewaxing, hydrofining to reduce sulfur content). This processing produces mineral oils with sulfur levels typically greater than 0.03% with viscosity index (VI) of less than 90.
- Group I+: Still have high sulfur and low saturates, but processing conditions have been adjusted to make higher VI. This higher VI, in the range of 100-105, gives better cold cranking and Noack performance, enabling these base oils to be used in 10W-30 engine oils with minimal Group III or Group II+ correction fluids.
- Group II. Mildly hydrocracked mineral oils with conventional solvent extraction of aromatics, solvent dewaxing, and more severe hydrofining to reduce sulfur levels to less than 0.03%, as well as removing double bonds from some compounds. Viscosity index is approximately 90-100.
- Group II+: There is now an informal Group II+ (not an official API definition) which emerged out of the need to describe base oils with a meaningfully higher viscosity index than the 100 than is typical of most Group II base oils. Group II+ base oils, produced through altered refinery processing conditions, typically have VI in the range of 108 to 115. These base oils offer performance advantages over Group II in some passenger car motor oil applications, specifically related to balancing volatility with low temperature viscometrics.
- Group III. Severely hydrotreated mineral oils with sulfur content between 0.001 and 0.01%; VI in excess of 120. These are considered VHVI (very high viscosity index) mineral base stocks. They behave like synthetics in VI but have much higher pour points (-20C versus -54C for PAO) which must be modified with pour point depressants for lower temperature use. They have lower oxidation stability than Group IV or V oils. These are made in proprietary processes by Chevron, Shell, Exxon, and others.
- Group III+: New Gas-to-Liquid base stock lubricating oils are being developed for market introduction in 2005 that will compete with Group III and PAO at costs similar to Group I. These will enter the passenger car motor oil market in response to demands for more 0W oils. They are created using catalysis of natural gas and have properties similar to PAO. They will have VI's exceeding 140 and will be used for 0W-XX and 5W-XX engine oils and super-premium transmission fluids.
- Group IV. Poly-alpha-olefin (PAO) synthetic oils with VI of 125-150. PAO significantly outperforms Group III in low temperature applications without the need for pour point depressant additives.
- Group V. Everything Else: Esters, polyethers, polyalkylene-glycol (PAG) synthetic oils with various VI ranging between 100 and 175. Group V can include quite low quality base stocks, like naphthenic base oils as well as very high quality base stocks like esters
- Esters are hygroscopic, causing water to enter the oil; and reactive, adding natural detergency, seal swell, and additive solubility to oil characteristics.
- [Europe Only] Group VI oils. These are polyinternalolefins (PIO) somewhat similar to PAOs. These oils are not readily available at present anywhere outside of Italy.
2. Synthetic Base Stocks. Group IV base stock is made of PAO and was for a while the only base used in synthetic oil until Group V (Esters) came along. PAO has a property of shrinking rubber gaskets, and when older cars were switched the leaking was generally due to this fact. Cars with new gaskets that used PAO-based oils from the beginning did not encounter shrinkage or leakage. The newer and the better synthetic oils began to incorporate a new base stock (group V -Esters) that helped to keep the rubber from shrinking. True synthetics are generally Group IV (PAO) mixed with Group V (Ester) base stocks, like Amsoil. Mobil began using straight PAO, switched to a very good blend of PAO and ester, then in their new Supersyn went back to all-PAO. Their additive package seems to be among the best. [Email from Mobil Products: Mobil1 Supersyn motor oil is not a hydrocracked oil, it is a group IV basestock which is a PAO base motor oil. It also has proprietary anti-wear synthetic package which is a group V basestock. The Mobil1 Supersyn is a fully synthetic motor oil with no petroleum additives.] [Comment from Geoff Williams: The first component (alkylated naphthalene) has the advantage over PAO and esters in that it has the best additive solubility and the best seal compatibility of the 5 most common engine lubricants (PAO, esters (2 types) and mineral oil). This is great news for older cars, with brittle seals that might be more suceptible to shinking with a PAO and ester-only based synthetic motor oil.]
3. Is it a "True Synthetic"? Prompted by a challenge to Castrol's use of hydrocracked Group III base oils in its Syntec product, the National Advertising Division of the US Council of Better Business Bureaus in 1999 allowed the definition of "synthetic lubricant" to include Group III-based oils. This decision resulted in a quick replacement of PAO by Group III oils due to $1.50 to $2 per gallon cost savings: most oil producers (with the notable exception of Mobil) made the switch in their synthetic products. There is some controversy about claiming VHVI Group III base oils as "synthetic". According to an unnamed PAO expert quoted in Lubricant World magazine, "The quality of Group III products is inconsistent, and their physical properties are different from one manufacturer to the next." Marketers using G III VHVI base oils in their "synthetic" lines include Castrol (Syntec), Valvoline, and Petro Canada. Those using Group IV or V oils include Mobil. According to Lubricant World magazine, "The synthetic market in general has seen ... new blends, new product releases, and formulation changes" and this continues to the present. Because marketers do not freely disclose oil formulations, caveat emptor prevails.
4. Oil Longevity. Synthetics are designed to last longer than conventional oils. Changing the oil every 3000 miles with synthetic oil or even Castrol Syntec is a complete waste of money. IMPE, using Castrol Syntec (what was provided during the free service period) in my VW TDI Turbo Diesel for 10,800 miles (the recommended change interval) it was found through oil analysis of the oil taken out of the engine when it was changed that it hadn't broken down, and the additives were not depleted enough to warrant changing. That was after nearly 11,000 miles in a turbo diesel. Your Volvo puts less demands on an oil than my TDI running up here in Michigan at below freezing temps, and the oil still lasted that long. A 3,000 mile oil change does not make sense for any car unless you are racing it or you drive less than 10,000 miles a year. For $15 you can have your oil analyzed to determine if you are changing it too soon or waiting too long.
5. API Standards. The standards set by the API are very easy to meet. Look for the Chevy Corvette standards (GM 4718) if you want a good oil. Or if you want the best protection against wear use an API CH-4 rated synthetic oil. Anyone can make an oil meet most standards, but the standards don't look at the amount of caking or sludge deposits. The additive package and the Viscosity Improvers have more to do with longevity and do not affect the passing of tests but do affect the long term health of your engine.
6. ILSAC Standards. The latest standard from ILSAC is GF-4, a 2004 standard developed to meet tougher emissions and fuel economy standards mandated by the U. S. Government. Automotive OEM's need better fuel economy to meet Corporate Average Fuel Economy (CAFÉ) limits. They also need their catalytic converters to provide reduced emissions for 120,000 miles. Higher quality base oils are an important part of the solution to GF-4.
7. Viscosity. Per the SAE (Society of Automotive Engineers), viscosity is a measure of an oil's thickness, or resistance to flow. Lower numbers indicate thinner oil and higher numbers indicate thicker oil. There are two types of motor oils, single grade and multi-grade. Multigrade oils such as a 10W-30 are designed to have the viscosity of an SAE 10W oil at cold temperatures combined with the viscosity of an SAE 30 oil at engine operating temperatures, The W or Winter designation indicates that the oil meets viscosity requirements for low temperatures (below 30°F). At the Chevron site http://www.chevron.com/ there is a nice SAE Viscosity Grade guide.
As an illustration, the Kinematic Viscosity is measured in centistrokes, the higher the number the thicker the oil (65 is thicker than 55) The Viscosity Index can be interpreted as an indicator of how thick the oil is, the LOWER the number the thicker or more viscous it is. The Viscosity Index of the base oil is a good measure of the oil's quality between 40 and 100 degrees C. At lower temperatures, some base stocks (Groups I-III) require pour point depressants and viscosity improvers which can break down over time. Hence, in comparing two base oils with the same VI, the better oil is the one with a lower pour point.
The following is for a 5w30 and a 0w30 made by the same manufacturer (synthetic based oils: Group IV and V blend)
Kinematic viscosity @ 100C cST (ASTM D-445 test) 11.5 and 11.3
Kinematic viscosity @ 40C cST (ASTM D-445 test) 66.00 and 57.3
Viscosity index (ASTM-D2270 test) 170 and 196
Now if we compare Castrol and look at the 5w30 compared to the 10w30 (Group I base stock nonsynthetic:)
Kinematic viscosity @ 100C cST (ASTM D-445 test) 10.7 and 11.3
Kinematic viscosity @ 40C cST (ASTM D-445 test) 63 and 80
Now in the same line of oils, a monograde SAE 30:
Kinematic viscosity @ 100C cST (ASTM D-445 test) 11.2
Kinematic viscosity @ 40C cST (ASTM D-445 test) 93
Quite a bit of difference there.
Here is some data from Chevron on their Supreme Motor oil which is all Group II base, just like Syntec except they also sell a true synthetic (numbers below are for 5w30 and 10w30 and 30, respectively):
Kinematic viscosity @ 100C cST: 10.4 and 10.4 and 11.5
Kinematic viscosity @ 40C cST: 62.5 and 69.8 and 10
Viscosity index: 155 and 135 and 101
Again the oil is thicker as the first number increases. Here is QuakerState:
5w30 and 10w30 and 30 (all Group II base, just like Syntec except they also sell a true synthetic)
Kinematic viscosity @ 100C cST: 10.7 and 11.0 and 11.2
Kinematic viscosity @ 40C cST: 67 and 73.2 and 90.5
Viscosity index: 155 and 140 and 113
Here is Pennzoil:
5w30 and 10w30 and 30 (all Group II base except for the straight 30 weight which is a Group I or solvent refined oil)
Kinematic viscosity @ 100C cST: 10.5 and 10.5 and 11.5
Kinematic viscosity @ 40C cST: 60 and 67.0 and 98Viscosity index: 160 and 140 and 105
Remember that the 5 is just for start up and gives you better protection if you start you car often, and the last number is what your car sees in operation, But it is possible for a 30 to be almost as thick as a 40 weight oil. The 30 will in some cases give you better fuel mileage than a 40 weight.
The heavier weight synthetics are designed for cars that are burning or leaking oil not for properly running cars. There is a market because some folks have been running synthetic and recognize how superior it is but wind up with a slight oil leak. Switching to a heavier weight synthetic will help reduce oil consumption. But using a 5w50 has some other problems besides creating a thick film ofhorsepower-robbing resistance, it stretches the limits on stability over time. The viscosity improvers needed to have a 5w50 (the w stands for winter) displace some of the lubricating molecules and are the first component to break down and create deposits in your engine.
Volvo does not recommend a single grade motor oil; they recommend multi-grades for their engines. Single grades should only be used as per manufacture's recommendation.
Viscosity Index improvers are mentioned at Lubrizol in a New York taxi test: http://www.lubrizol.com/ This test was much different than the Consumer-sham-Report/Review test on NYC taxi cabs, the results are more meaningful. In summary, the New York City taxicab fleet test provides persuasive evidence of the outstanding performance of Lubrizol viscosity modifiers. Despite the severe operating conditions, Lubrizol viscosity modifiers, combined with the Lubrizol performance package, provided superior engine cleanliness and durability. Further, they maintained their remarkable rheological characteristics over the extended drain intervals of the test, providing consumers with additional confidence that their cars will start and operate reliably in all weather conditions.
8. Break-In Period. The issue of using a dino/mineral oil as a break in oil is somewhat overblown. And since you are using Syntec, you are still using a mineral oil, NOT a synthetic oil. Like I said before, Syntec is a highly refined and stabilized MINERAL oil, it is not a synthetic in the terms you are thinking. Regular oils, Dino - mineral - petroleum - whatever you call them, are most commonly solvent refined, a process that leaves many impurities in the oil. Castrol and many other companies now are using the hydrocracking process that refines the crude oil without using solvent separation, resulting in an ultra clean pure product. BUT IT IS STILL A REGULAR OIL. If you want to switch to a synthetic oil wait till you have 10-20k miles on the engine. At that point your car should be 'broken-in' enough for you to feel comfortable about using a REAL synthetic.
A couple comments about break in myths and synthetic oil consumption after switching: Most break-in periods in new engines will be accompanied with some oil consumption. If this is not the case for a particular engine switching to synthetic early will not prevent normal break-in. The extra slipperiness of a synthetic might prolong the period but it will provide extra protection and as long as you don't beat up the engine by over revving it too much or loading the car up and climbing a mountain before it is broken in you should be fine.
9. Switching to Synthetic Oil. As far as switching from normal oil to synthetic I think that it is better to change over all at once, but you will need to make two or three changes before resuming or beginning normal change intervals. I would put a full crankcase of synthetic in, drive it for 1000 miles, change the filter, drive 2000 miles and change the oil and filter, and then change both after 3000 miles then begin either 5, 7.5 or 10k mile oil change intervals. The filter changes are important because the new oil will be removing lots of deposits and sludge and you don't want to overload a filter. The extra cost and time spend with the first few changes will be rewarded with a smoother running car, lower costs (extended drain intervals, and if the proper viscosity is chosen fuel savings) and a nice clean running engine. [Added Remarks from Mr. Lube] The synthetic will clean a great deal of gunk out, which will plug the filter quickly. I have seen it happen at my oil change shop over and over and over. I tell people to bring the car back after a month to change the filter only. It can and will cause some damage. I do not think it is a matter of preference or choice really. Rather, the $4.00 filter will become plugged with the switch, and it is left to the wise to follow past experience and advice from the ample evidence. Get it changed, and you will be surprised how dirty the filter will be.
Check out these sites for more info on oil:
- Lubrizol Lubricant Data and lubrication theory: http://www.lubrizol.com
- Mobil and Chevron
10. Fuel Economy Issues. See Lubrizol's discussion of the effects of base oil on fuel economy at: http://www.lubrizol.com
[Williams:] My personal experience is in line with this and I have seen an increase in Fuel Economy of 10% (24 mpg to 26.5 mpg) since switching from a Group I 10W-30 to a Group III/IV/V 0W-30 blend.
11. Synthetic ATF. [Editor] In the Lubrizol knowledge Base site at www.lubrizol.com, they note that two European commercial vehicle automatic transmission makers have posted specs for mineral oil versus synthetic automatic transmission fluid lifetimes. Voith allows 60k km drain intervals on mineral oil and Group III (hydrocracked semisynthetics) ATFs and 120k km intervals on full synthetics, both in Dexron III specs. ZF allows 30k km for mineral oil, 60k km for part synthetic, and 120k km for full synthetic, again in Dexron. This is an indication of the value of synthetics in normal use. Mobil 1 ATF is a full synthetic. [Editor] Note that the new DEXRON-VI specifications result in improved performance: fluid life is significantly longer due to improvements in oxidation stability, friction durability, shear stability and foam resistance over DEXRON-III fluids. DEXRON-VI is backwards compatible for Volvo cars.
 Oil Filter Information Links.
[Tip] For a comprehensive study on common brands
of oil filters, see:
Oil Filter Information Links.
[Tip] For a comprehensive study on common brands
of oil filters, see:
http://minimopar.knizefamily.net/oilfilters/index.html
This study was written by Russ Knize and may be found, if the link is broken, by searching on "Minimopar" and "oil filter".
For information on filters and filtration media, see: http://www.filtercouncil.org and look for "technical bulletins."
Kane Leung deconstructed a Mann W-917 filter, used on B230 series engines, to find:
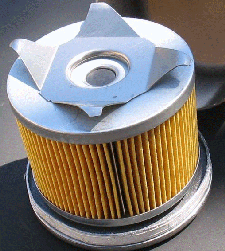 Overall inner dimension of 3-1/4" diameter.
Overall inner dimension of 3-1/4" diameter.- Metal caps on both ends (unlike Fram which uses cardboard).
- An anti-drainback check valve, consisting of a full circumference nitrile rubber flap.
- A strong, spring loaded bypass valve with nitrile rubber seal.
- 90 pleats, pretty evenly spaced, each glued down very tightly. The paper is ribbed to keep the pleats separate.
- The filter pleat measured 2-1/4" tall, with 89 of the pleats measuring to 25/32" deep. The pleat at the crimp measured to 3/4".
- Each pleat offers 2 sides, for a total of 180 sides of filter surface. (2.25*0.78125*178) + (2.25*.75*2) yields just over 316 sq.inches. By comparison from the other oil filter study out there, the gigantic Ford/Motorcraft FL-1A offers 400 sq.inches of filtering area. In this same size, the Fram PH8 offers just 193 sq.inches. With the Volvo filters being shorter, you can imagine how little filtering area the Fram will offer.
Oil Additive Information Links. Thinking of using Slick 50 and other additives? The Federal Trade Commission had a case on Slick 50 which denied most of their advertising claims. Overall good advice is: always use high-quality lubricants at the proper viscosity range; never use additives, especially PTFE-based oil enhancers.
Fuel:
Gasoline Recommendations for Turbo Engines.
[Inquiry] What octane requirements work best with a turbo? Around here, 90 grade with ethanol is widely available; 92 is more expensive; Will 87, 90 or 92 work?
[Response 1: Bernard] The B230FT engine is set up for the North American market to run without problems on 87 octane engine (ROZ = MOZ :2). In today's view, that might not have been the wisest decision. Most drivers would appreciate a few horsepower more (maybe on B230ET-level) and rather spend 10 cents a gallon more on gas. Anyway, if knocking is detected on a late model 700 or early 900 car, the Bosch Motronic will retard ignition automatically. However, as with many other cars from other manufacturers, it might very well be that someone experiences knocking when buying no-name 87 octane gasoline. Many of turbo Pilots have therefore decided to run at least 89 octane, many even 91octane gas all the time, myself included.
[Response 2: Phil] I'm stuck with that ethanol gas also. I can't run 89 so I'm stuck with the more expensive 92. When I can get undiluted gas it makes a noticeable difference in performance. If you can't get anything but the ethanol you'll probably need to use the 92. [Editor's note: using lower octane will cause retarded timing, lower power and higher engine operating temperatures. Use your own judgement.]
Reformulated (Oxygenated) Gasoline Areas. This map from the EPA shows reformulated gasoline areas in the US: Several reports have been received about poor quality reformulated gas causing valve seizures in B230 and B6300 engines driven on short trips with old gasoline.
Replacement for Leaded Fuel. [Editor] If you have an older engine (e.g., many EU cars through 1989) requiring leaded fuel, now unavailable in many countries, check this link from the UK Volvo Club for information from Volvo about dealing with your fuel needs.
Sulphur or "Rotten Egg" Odor. [Excerpt: Volvo Tech Tip 4/6] Hydrogen sulphide is created in the catalytic converter from sulfur in fuel. Its likelihood increses during short trips in stop-and-go traffic, hot climates, and winter fuel formulations. A sulfur concentration of 0.03% can cause the odor. Many regular-grade fuels contain well over 0.1% Replacing the converter will not solve the problem: only a low-sulfur fuel such as premium grade will work.
Car Storage, Fuel Degradation and Engine Deposits. [Based on Tips from Chris Herbst] If you store a car for long periods, be sure to use a fuel stabilizer to eliminate varnishing and deposit formation. There is some evidence that use of ethanol-based fuel in stored cars will result in serious valve damage: the fuel tank is coated with gummy precipitate, valve stems gum up with deposits and the head requires complete cleaning. See Car Storage Tips